Alternative solution to diverting stoma
Alternative solution to diverting stoma
Overview
Most colorectal surgeries include the formation of a colorectal anastomosis. One of the most dangerous complications related to the anastomosis is anastomotic leakage, Occurring between 5 and 20%. Diverting stoma is the standard of care used to reduce morbidity associated with anastomotic leaks, but it involves a high rate of complications associated with the stoma itself along with patient discomfort, reduced quality of life, increased hospital stay, additional surgery, and increased medical costs.
New approaches are needed to satisfy the unmet needs for a short- term temporary bypass of low rectal anastomoses, in patients with a high risk for anastomotic leak.
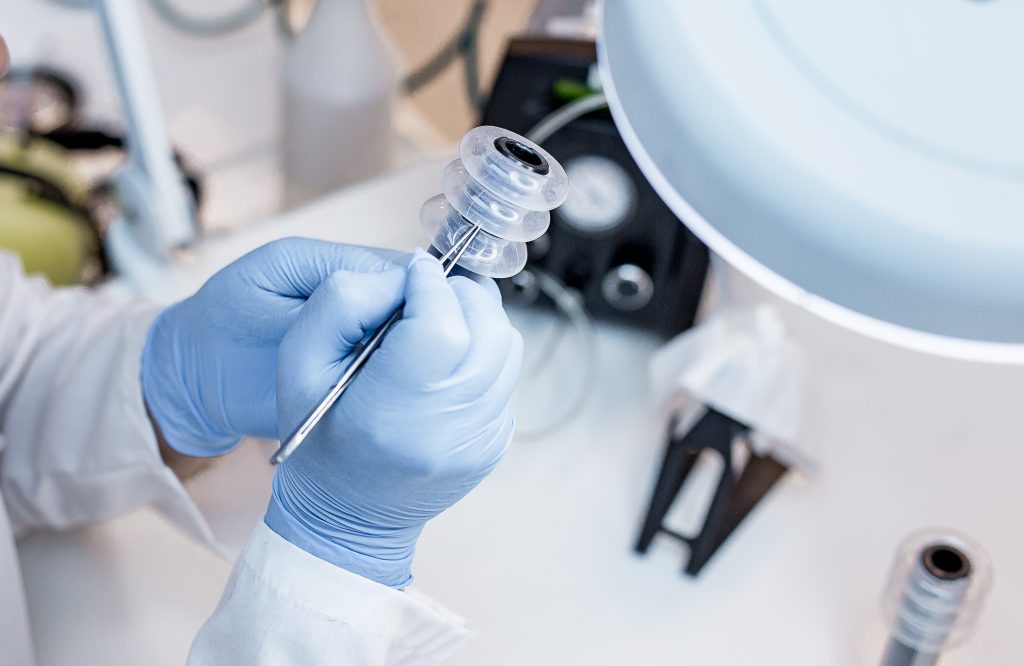
CG-100 Intraluminal Bypass Device, CE marked product, is a silicone tubular sheath that is introduced into the lumen of the intestinal tract through the anus, using a designated delivery system. The sheath is positioned proximal of the anastomosis. At the distal part of the sheath there are 3 inflatable balloons. The sheath is held in position by an extra-luminal ring which encircles the colon.
The diameter of the balloon is slightly larger than that of the ring, preventing the sheath from moving downstream beyond the ring location, while still allowing it to move freely inside the colon, preventing damage to the colon wall.
After ten days, when the risk for anastomotic leakage is reduced, the sheath is removed without any additional surgical intervention. Extremely simple to apply and remove, it can be extracted at any time, requiring only a few minutes.
Please read IFU before use and for additional info on indications, contraindications, warnings and precautions.
The CG-100 clinical study is underway at select sites.
If you have patients who would like to be considered for the study,
they can visit : http://stomachoice.com/apply-now/

