

Transforming
Colorectal Surgery
Transforming
Colorectal Surgery
Colospan offers a unique alternative to the diverting stoma by providing a temporary intraluminal bypass which helps reduce the contact of fecal materials with the anastomotic site. This temporary diversion solution is removed without surgery in under two weeks and aims to minimize post-operative complications for a comfortable recovery.
CG-100 is a temporary intraluminal bypass device designed to reduce the rate of diverting stoma and their related complication. CG-100 divert fecal material from coming into contact with the anastomotic site during its critical healing period thereby reducing the life-threatening symptoms related to clinical anastomotic leaks.
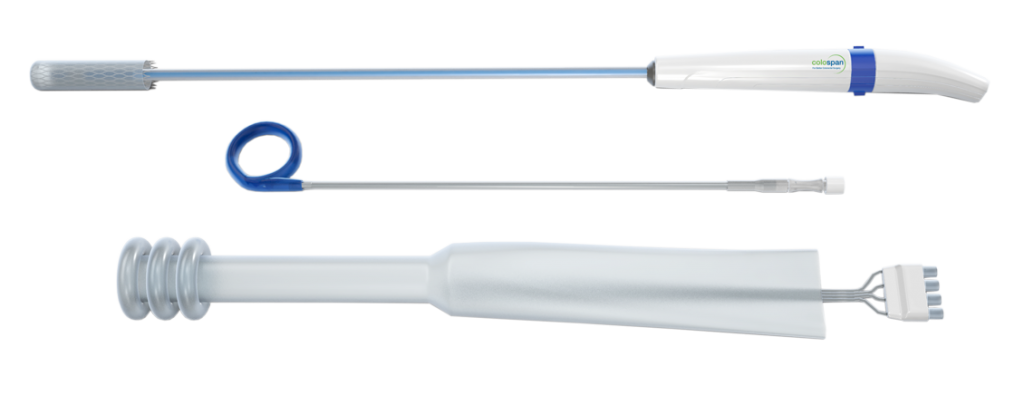




The CG-100 clinical study is underway at select sites.
If you are a rectal cancer patient between the ages of
22-75 planned to undergo a colorectal surgery
and would like to be considered for the study, click here.
What We Do
Colospan Ltd has received funding from the European Union’s EIC Accelerator program,
Project # 190124297 – CG-100
Disclaimer: CG-100 received European CE mark in 2014 and is available in the European Union for commercial use, CG-100 is not approved for sale or distribution in the US and is limited by U.S. law to investigational use.
Please read IFU before use and for additional info on indications, contraindications, warnings and precautions.