Kfar Saba, Israel, January 8, 2020 Colospan which develops novel solutions for colorectal surgery, announced today that the United States Food and Drug Administration (FDA) has approved the company’s investigational device exemption (IDE) application. With this IDE approval in hand, the company will launch its pivotal study for CG-100, a temporary Intraluminal Bypass Device, designed to reduce diverting stoma rates in patients undergoing gastrointestinal resection procedures.
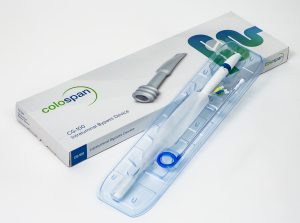
Anastomotic leak is the most devastating, feared and common complication in colorectal surgery. When a leak occurs, fecal content can enter the abdominal cavity resulting in potentially serious effects to the patient. Diverting stoma is the current suboptimal gold standard used by surgeons today to avoid the clinical consequences of anastomotic leaks in patients undergoing colorectal surgery. CG-100, Colospan’s lead product, was developed as an alternative approach to diverting stoma. In comparison to a diverting stoma which is usually deployed for 4-6 months and requires a second surgery for removal, Colospan’s CG-100 is deployed for 10 days and can be easily removed under x-ray without the need for a secondary surgical intervention.
Colospan will launch a prospective, randomized pivotal study in Q1 2020, which will be conducted at leading medical centers in the US and Europe and is intended to evaluate the safety and efficacy of the CG-100 to support future regulatory approval in the United States.
Boaz Assaf, Chief Executive officer of Colospan, stated: “We are pleased to have received FDA approval of our IDE application and we look forward to initiating the pivotal study which is a leap forward towards bringing our novel device closer to the market. The pivotal study will allow us to assess the potential of our device to improve patients’ lives, significantly reduce healthcare costs and as a result empower physicians to deliver an improved standard of care. This is a significant milestone for our company as we advance the clinical development of our novel technology in the U.S.”
About Colospan
Colospan Ltd. is a medical device company focusing on the development of novel and proprietary solutions for colorectal surgery. The company is dedicated to addressing the clinical and economic consequences of anastomotic leaks, the first and foremost challenge in colorectal surgery. Colospan’s lead product, CG-100, is designed to offer an alternative to the ostomy pouch, to minimize health risks and enable patients to maintain dignity post-surgery. Colospan’s team consists of seasoned professionals in marketing, sales and development of surgical devices for colorectal surgery, supported by key opinion leaders (KOLs) from Europe and the United States. The company holds a CE mark for CG-100 intraluminal bypass device as well as ISO 13485:2016 certification. The CG-100 is not approved for sale in the United States and is limited to investigational use.
