The CG-100 clinical study is underway at select sites across the U.S.
If you are a rectal cancer patient between the ages of
22-70 planned to undergo a colorectal surgery
and would like to be considered for the study, click here.
Introducing CG-100
Specifically designed to create a temporary intraluminal bypass
Aims to reduce the rate of diverting stoma by reducing the contact of fecal material with the anastomotic site
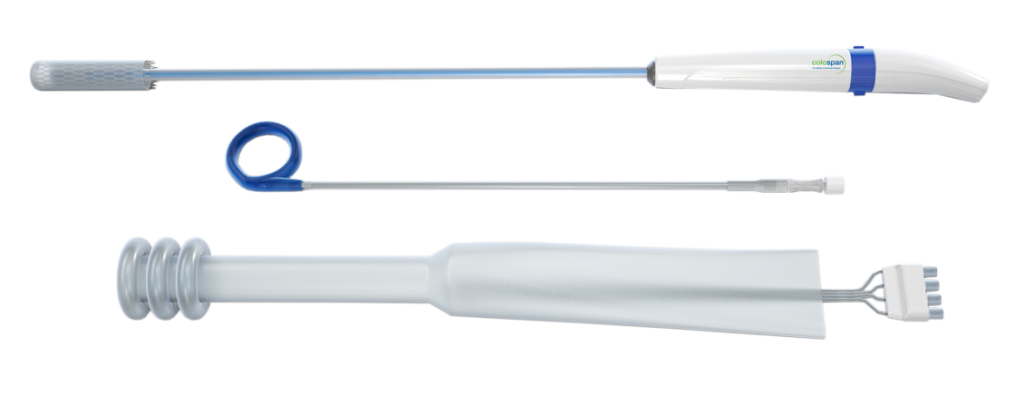
CG-100 is limited by U.S. law to investigational use and is not approved for sale or distribution in the US
Contact us
TEL: +972-9-7672518
FAX: +972-9-7672498
EMAIL: info@colospan.com
ADDRESS:
U.S. office location at 125 Cambridge park Drive, Suite 301 Cambridge, MA 02140
Israeli office location at 21 Atir Yeda St, Kfa Saba, 4464316 Israel
